Okay, so preface here – I am not a scientist. I am neither very knowledgeable about science, nor am I terribly interested in its nuances, but 18th Century scientific discoveries are today not extremely advanced stuff, so I feel more or less qualified to talk about this, having taken high school chemistry. If I say something incorrect or simply imprecise, feel free to correct me in the comments.
Anyway, Lavoisier. As you can probably tell from the name, he’s French. He’s best known for his work as a chemist, but he did a lot more than just that. Lavoisier began to work on the actual mechanism behind respiration and was one of the first to connect it to metabolism. He was an activist, attempting to use his own prestigious position within France to save foreign academics from the Reign of Terror, and even had a part in creating the metric system.
So, we’ll start at the beginning with Lavoisier’s education, which was as a lawyer. He practiced relatively little law, as you might imagine, and at the age of 26, in 1794, he helped publish the first geological map of France. After this, he was elected to the French Academy of Sciences, the prestige of which is somewhat difficult to understand in today’s academic system. Today, scholars of all sorts may talk to one another via email or video-chat without much problem, and they can easily and readily view one another’s work in academic journals. As a result, many academics have myriad opportunities to collaborate with minds of their same caliber. In Lavoisier’s day, such an opportunity was almost unheard of. With the exception of perhaps the British Royal Society, there existed no other entity in 1769 that included so many learned men, almost all of whom had given valuable contributions to the sciences. There were a few honorary members (Napoleon, for instance), but the level of academic discourse at the Academy must have been almost foreign to these men, who would have been used to having to dumb down their work for others so that they could be better understood.
Around the time of his admission into the Academy, Lavoisier bought a share in the Ferme générale – essentially a privatized form of the IRS used by the French Monarchy in the 18th Century. This gave Lavoisier a certain standing within the government, which he attempted to use on monetary reform, which would have helped the peasants, but ultimately, he was ignored.
In 1772, Lavoisier began devoting time to working on Phlogiston Theory – which posited that all flammable substances contained a chemical called Phlogiston, which, when burned, would release part of itself into the air (smoke) and would also disintegrate into a solid (ash).
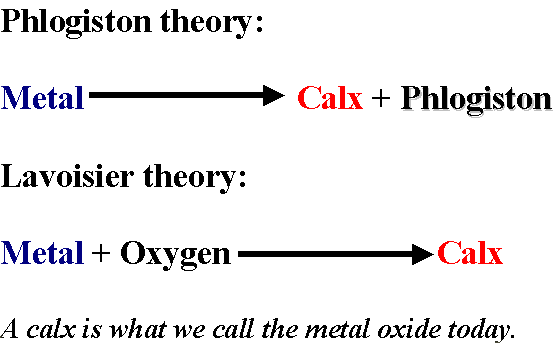
Lavoisier’s experiments noted that after burning substances, somewhat counter-intuitively, weighed more than before combustion, but phlogiston theory would suggest that with the escape of the now-combusted phlogiston, the burned object would weigh less. Lavoisier presented his findings to the Academy, where Joseph Priestly, a visiting Englishman, heard Lavoisier’s thesis. Priestly and Lavoisier worked together for a time on the issue, and Priestly came to call what we now know as Oxygen “dephlogisticated air.” In 1783, Lavoisier read to the Academy his new paper Reflections on Phlogiston, in which he launched his attack on Phlogiston Theory as a whole, taking things a step farther than Priestly had.
Teaming with Pierre Simon de Laplace, another famous scholar of the time, Lavoisier went a step further and attempted to see what would happen when one took this new gas, which was somehow linked with combustion, and combined it with another newly-discovered gas, which Lavoisier named “Hydrogen” (literally water-maker; nobody ever called scientists creative at naming things). When the two reacted, they produced water, and so, Lavoisier and Laplace found that they had accidentally proven that water was not an element – a belief that had been held for 2,000 years. Oops.
Unfortunately, nobody could agree on how this finding affected combustion theory. Lavoisier would contribute a few other things to combustion theory, such as the fact that amplified sunlight could accelerate combustion, but there was still no consensus on the actual mechanism of combustion and how it works.
Lavoisier would move on to work on Stoichiometry, which he is often erroneously named the “inventor” of. Number one, he didn’t invent it, he discovered it. Number two, people had been making stoichiometric observations for well over a century at the time of Lavoisier. What makes Lavoisier unique is that he seems to have been the first to understand the whole process mathematically. Mikhail Lomonosov had stated half a century before that nothing is actually lost in a chemical reaction, but Lavoisier turned the whole thing into a simple algebra problem. He also attempted to form a sort of periodic table, which he was never able to perfect as completely as he wanted. It consisted of some 55 elements, which he could not distill any more than he already had, and in 1789, he published the first actual textbook on chemistry.
Other notable observations and inventions of Lavoisier were that diamond is simply another form of carbon, the most efficient form of saltpeter production to date (which was key in producing gunpowder), and a new method for lighting the streets of Paris.
After the French Revolution broke out in 1789, Lavoisier began to tread more carefully. He worked with Laplace once more to begin creating the national set of weights and measures demanded by the new French Republic, but he entered suspicion when he began defending foreign scientists who were working in France at the time. Lavoisier was already distrusted by the regime, due to his work with the Ferme, since taxes were in large part viewed as a royal tool to repress the people. Moreover, much of Lavoisier’s work had been enabled by the government. In the 1770s, he had been requested by the crown to oversee gunpowder production for the French army, a job which Lavoisier seems to have been very successful at. Moreover, Lavoisier’s position gave him a lab in the National Armory, where he could conduct scientific experiments of his own on the government’s dime. In November of 1793, the order of all former tax gatherers was ordered, and Maximilien Robespierre named Lavoisier a traitor not long afterward. He was executed by guillotine on May 8, 1794, shortly before Robespierre fell from power and was himself guillotined.
Lavoisier was but one among several scientists executed during the Reign of Terror, which greatly contributed to France’s “brain drain” in the early 19th Century. Although the effects of this phenomenon on the French economy are hard to quantify, France would never be truly competitive with Great Britain economically throughout the 19th Century. The historian’s job is not to answer “what if” questions, but I do not believe it is unreasonable to say that had France not killed so many of its educated people during the Reign of Terror, it might have had a better economy. That seems like pretty simple logic.
Anyway, I hope you enjoyed this piece. Lavoisier is an incredibly diverse guy, and the basis of the way we study chemistry today is largely due to his (and a little bit of Laplace’s) work. I’m not a big scientific historian, but the occasional guy is pretty interesting, so later on, I might throw out a piece like this again.
Thanks for reading,
Paul

